|
Home
The Rockbridge Bloomery –
Reports
Smelting Enriched Bog
Ore in a Low Shaft Bloomery.
Jonathan Thornton, Skip
Williams and Aaron Shugar
Introduction:
During the 3rd
International Symposium on Early Iron, held at Eindhoven, Holland, I participated in an iron smelting experiment along
with Skip Williams, also of the United States,
and with the occasional assistance of other casual helpers. I am a
conservator and professor of conservation, as well as a blacksmith and tool
maker. Skip Williams has extensive experience in smelting iron using small
furnaces, though usually, these employ modern materials. The aim of our experiments
was to produce a good quality bloom based on the experience of Skip Williams,
but using technology that more closely approximated historic models than do
the majority of his smelts. The furnace that was built survived five smelts
in good condition, and did indeed produce a high quality soft-iron bloom with
some superficial steeling. The following description will make clear
both how the experiment attempted to duplicate the evidence of archaeology,
as well as how it intentionally diverged. Analysis of the resulting bloom was
carried out by Dr. Aaron Shugar, Mellon Professor
of Conservation Science here in the Art Conservation Department at Buffalo
State College.
 Furnace: Furnace:
The furnace was constructed
from a sandy, iron-rich clay (loam) mixed with straw. This can be thought of
as a low-firing ceramic body. The role of sand and other inorganic “tempers”
in such a body, whether it is used for pots, tiles, or furnaces, is to
decrease shrinkage by reducing the ratio of the highly shrink-prone clay as compared
to other non-shrinking components. Grog (pre-fired ceramic) has also been
used in the composition of furnace material (Crew and Charlton 2005), and for
the same reason. The role of the straw is to increase the strength in
un-fired material by binding it together internally, and to provide vent
ducts for the escape of steam and other gases as the material converts into
ceramic. It may also play a role in stopping the propagation of incipient
cracks. This has been a common strategy in low-firing ceramics.
Examples of the material from the destruction of similar furnaces at Eindhoven show that the inner
sections are well supplied with empty tubes from the straw having been turned
to ash, while outer sections contain straw that is not even charred. As would
be expected, the furnace fragment exhibited a dark grey
reduced-iron zone on the interior, and an abrupt transition to red oxidized
material on the exterior zone.
The site chosen for the
furnace was near a previous furnace that was mostly demolished. The remains
of this furnace formed a small mound, into which the circular shape of the
new furnace was marked out and excavated into the slope to a depth of only a
few centimeters at the front where the tap arch would be located. The
bottom of the hearth was roughly leveled and compacted.
The furnace material were
prepared by treading the damp loam and additional water with the chopped
straw (using bare feet), until it had a firm, plastic consistency. This
material was formed into ovoid “bricks” of about the size and shape of small
loaves of bread. These were then used to build up the superstructure of the
furnace. The areas opposite the tap- arch that were excavated into the mound
were lined with fragments of fired pots so that the wet ground would be
better insulated from the furnace.
The interior space of the
furnace was reserved and determined by a bundle of reeds that the museum had
available for thatching purposes. Owing to the natural taper of individual
reeds, the bundle also tapered when the bundle was set upright on the cut
ends. This provided an advantageous inward taper to the smelting
chamber. Reed impressions have been noted before in archaeological
investigations of furnaces (Crew and Charlton 2005). It had been supposed by
these authors that the reeds had been burned out prior to heating the
furnaces. We may have tried this also had the museum not wanted them
returned. As it turned out, the reeds were easy to pull out once the furnace
had become somewhat firm. This was done by pulling the center reeds out
first, and working to the outside. Once a furnace has been fired, it is
difficult to tell whether reeds were removed intact or by fire, except
perhaps by smearing of the impressions.
Although the interior space
was reserved by the reeds, the exterior of the wet furnace material did not
support itself well as the furnace gained in height. It appeared to exhibit
the property known as thixotropy- that is that it
flowed under applied force (such as patting and kneading). Some Eindhoven participants solved
this problem by building a wood-fire in the interior, so that the lower
portions would dry and become more firm as construction proceeded. As the
main fabricator of our furnace, it occurred to me that an outer frame-work
composed of handy and nearby willow shoots, would provide enough support to
allow construction to the full intended height. Unbeknownst to me at the
time, archaeological evidence has shown similar small “post holes” around
excavated furnaces (Crew and Charlton 2005). This was an interesting example
of convergent problem solving and the value of experimental
archaeology. This wicker-work cage was made by sticking the willow
shoots into the ground around the outer circumference, angling them inwards, and tying them together with cord as the
furnace gained in height. They supported the furnace well, and were clipped
off flush with the top of the furnace after the final course of bricks was
added, the reeds removed, and the outer and inner walls smoothed.
Interestingly, the wicker-work survived all four smelts mostly intact, a
testament to the insulating qualities of the furnace walls.
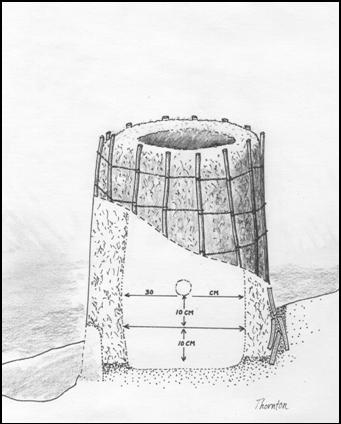 The tap arch was marked out and thinned on
the exterior front of the furnace (opposite the mound) and the tuyere hole
was located above and on the side, ninety degrees from the tap arch. Skip
Williams had previously determined that furnaces tend to thin out near the
tuyere. It was his intention to start with a thinned wall in this location so
as to keep that area somewhat cooler, and to prevent the bloom from adhering
to the tuyere. This was thinned from both the outside and the inside surface
by reaching down into the furnace. It should be noted that all areas of the
interior were reachable, aiding in modifications and subsequent repairs. The tap arch was marked out and thinned on
the exterior front of the furnace (opposite the mound) and the tuyere hole
was located above and on the side, ninety degrees from the tap arch. Skip
Williams had previously determined that furnaces tend to thin out near the
tuyere. It was his intention to start with a thinned wall in this location so
as to keep that area somewhat cooler, and to prevent the bloom from adhering
to the tuyere. This was thinned from both the outside and the inside surface
by reaching down into the furnace. It should be noted that all areas of the
interior were reachable, aiding in modifications and subsequent repairs.
Cracks did develop however- in
particular, two large vertical cracks opposite each other- one on the tap
side, and one on the back. This was alarming enough during the first smelt,
that an iron hoop was quickly fashioned and forced down over the furnace exterior
to prevent collapse. These large cracks were patched after the first smelt
and never opened up again. The interior of the furnace remained essentially
intact except around the tuyere area, which spalled
and thinned further. This was not repaired as it seemed to be what the
furnace “wanted to do,” in confirmation of the reasoning behind thinning this
area at the outset.
Before use, the furnace was
dried and pre-heated by building a wood fire in the interior and burning it for
a few hours. During this process, the entire furnace began to steam as water
was driven off by the internal heat.
Ore:
The ore that was available to
the Endhoven participants was a bog ore from North
Western Germany. Previous analysis carried out by Dr. Arne Espelund indicated that most samples were of relatively
low iron content, and high in phosphorus. Based on the quantity of iron that
would necessarily be reacted with the silicates and end up as slag, it was
his prediction that this ore should not produce much if any iron, despite
previously reported success.
As a means of enriching this
bog ore, Skip Williams requested a supply of hammer-scale (magnetite). This
was supplied by the museum. During the two successful smelts, the hammer
scale was mixed with and charged with the roasted bog-ore in a ratio of 50/50
by weight to simulate an ore of approximately 80% FeO
content. Whether or not such “enriching” materials would have been available
historically, or whether they even needed to be, is open to conjecture. It
does seem likely that all high-iron content waste from bloom consolidation
and smithing would have been recycled into a furnace, as perhaps also would
magnetic slag. Since most blooms would have been smithed
into useable bar iron on the site of the bloomery, the waste from smithing
would have been available to early smelters. The loss of weight from the
bloom to bar iron has been reported as high as three quarters of the original
bloom, to as low as twelve percent (Craddock 1995). In our experimental
smelts, the spongy reduced iron towards the outer portion of the bloom was
knocked off with a small hand hammer before consolidation of the remaining,
densely metallic bloom. This material too, could have been easily
recycled. Also, the bog ore supplied to us was not sorted according to
density and apparent quality, but seemed variable enough that careful
selection might also have increased the iron content.
Fuel:
In common with all
participants, the charcoal supplied was a hardwood charcoal produced using
traditional methods in Germany.
This was highly variable in the size of pieces. We reduced the size by
stamping on it and chopping it with shovels, then we sifted out the fine
particles (“fines”) using a coarse screen. Charcoal size is relevant to burn
rate, and it was the intention of Skip Williams to have charcoal chunks that
were no larger than a few centimeters, and without the fines that cause
excessive and annoying sparks. During the sorting process all pieces of
unburned wood as well as stones were discarded.
Draft:
Participants in the symposium
used a variety of blowing apparatus, both mechanical and human-powered. We
used an electric blower fan, reasoning that a blast is a blast, and having no
available slaves (or bellows for that matter). The air was forced through a
T-shaped piece of pipe so that the interior of the furnace could be observed
through a glass port in line with the tuyere. This arrangement also allowed
us to reach through the tuyere with a rod and clear slag drips from the
interior end when it seemed on the way to becoming clogged.
Tuyere:
In his previous smelts, Skip
Williams has used a tuyere that projects somewhat into the interior of the
furnace. He brought to the symposium a copper tuyere forged from a heavy
piece of copper plate with the intention of using this during the
experiments. This was used during the first smelt with success. The use of a
copper tuyere dictates that the blast must remain running during an
unclogging operation, or the end will quickly melt off. For this and other
reasons, we decided to fabricate a ceramic tuyere that could better withstand
smelting temperatures. Dry, white-firing primary-type clay was used for this,
mixed approximately 50/50 with sand, and kneaded to a plastic “potting”
consistency with water. I started fabrication by penetrating both thumbs into
the center of a ball of clay, then pinching out a tube of clay with my thumbs
still inserted- essentially creating a double “pinch pot.” I then inserted a
straight willow stick approximately the width of my thumbs, and increased the
length of the tuyere by working it out along the stick. I placed this over
the opening of the furnace that was still hot from the first smelt and
allowed it to become firm. Once it had done so, I tapped the projecting end
of the stick on a hard surface so that the tuyere would slide down and off. I
made two such refractory ceramic tuyeres during the
experiments. The first of these was pre-fired, but proved to be too short
(particularly after it was accidentally stepped on during the frenzy
attending the extraction of the first bloom). It was quickly replaced with a
longer one that was installed without pre-firing. This second tuyere survived
subsequent smelts essentially intact, with only slight vitrification
and slag erosion at the tip.
The tuyere was inserted at an
angle of approximately 20-25 degrees above the horizontal, an angle arrived
at by folding a piece of paper diagonally (45 degrees) folding it diagonally
again, and comparing the slope as the tuyere was sealed into the hole created
for it using soft loam. The end of the tuyere projected into the interior of
the furnace roughly as far as the original interior of the furnace before it
was thinned in that location. This ensured that the blast would penetrate
into the interior of the furnace, but that the tuyere would not be crushed or
clogged by the constantly settling charge. The blower pipe was inserted
into the flared end of the tuyere, and also sealed with loam.
Smelting:
The bottom of the furnace was
filled with charcoal fines to a depth that was just a few centimeters below
the tuyere opening. The fines would essentially provide an insulating support
for the slag and bloom. This bed would also be easy to excavate during bloom
extraction.
Hot coals were added, and the
charcoal fire started. Once it was burning well, the blast was turned on, and
the furnace filled with charcoal. With the furnace full of incandescent charcoal,
the charging was begun. The intent was to keep the furnace full of burning
charcoal and ore so that immediately after fresh charging, it was slightly
mounded above the top of the furnace. The ore was charged in back of an
imaginary line bisecting the circular top, and opposite the tuyere. In Skip
Williams’s experience, this allowed the ore to settle towards the tuyere as
it tends to do, but not so far as to block it with the bloom. The intent is
the produce a bloom in the center of the furnace. Charging with
charcoal and ore was repeated when the charge had sunk enough to accept a
fresh charge. In general, the furnace was charged with equal weights of ore
and charcoal at each charging, though the fuel-to-ore ratio was increased a
few times when, according to the experience of Skip Williams, the furnace
appeared to be burning too slowly. During most of the smelt, the furnace was
charged every fifteen minutes. The smelt was continued until the starting
quantities of ore had been exhausted, though further charges of charcoal were
burned after that, and before bloom extraction.
 Tapping and extraction: Tapping and extraction:
The blower was turned off, and
a rod was driven through the top of the tap arch until the pockets of slag
were found. This drained off into a previously prepared depression that had
been lined with a layer of charcoal fines. The slag was fully molten and
fluid, running out into quickly cooling blocks that were removed with a
shovel. This slag proved to be weakly magnetic, probably owing to the inclusion
of particles of reduced iron and/or magnetite. When the slag had been
depleted, the tap arch was gradually enlarged with a shovel and the location
of the supposed bloom was undermined to create a cavity that it could drop
into. During this process, incandescent charcoal poured down around the sides
of the bloom, and was also shoveled out. Finally, with the assistance
of a large wooden pole that was banged down on the top of the bloom from
above, the bloom dropped into the undercut cavity and was extracted through
the tap arch. The first smelt produced a solid bloom that was immediately cut
into two pieces with an axe and sledge. The second and larger bloom was
consolidated intact: First the spongy iron and slag was knocked off the
exterior surfaces with a hand hammer, and then the solid lump was
hammer-consolidated on a stump using heavy sledges (triple striking at one
point).
Magic:
 On an anthropological note, I was struck by
how similar the tapping and extraction process was to childbirth: The slag
pours out (“water breaks”), the tap arch (birth canal) is gradually enlarged,
the bloom descends (the birth canal) and is finally extracted (not without
difficulty) in an atmosphere of excited expectation and purposeful
preparation and activity. This could not have been lost on ancient people and
would no doubt have influenced and increased the significance of attendant
ritual and “magic.” We ourselves decorated the tap arch with a vulval symbol, fashioned a small Venus figurine from the
tuyere clay, and poured an “offering” of duty-free whiskey into the furnace
before charging began. It should never be forgotten in interpreting ancient
sites that such things were equally “practical” to early technologists as ore
and fuel quality or furnace design. On an anthropological note, I was struck by
how similar the tapping and extraction process was to childbirth: The slag
pours out (“water breaks”), the tap arch (birth canal) is gradually enlarged,
the bloom descends (the birth canal) and is finally extracted (not without
difficulty) in an atmosphere of excited expectation and purposeful
preparation and activity. This could not have been lost on ancient people and
would no doubt have influenced and increased the significance of attendant
ritual and “magic.” We ourselves decorated the tap arch with a vulval symbol, fashioned a small Venus figurine from the
tuyere clay, and poured an “offering” of duty-free whiskey into the furnace
before charging began. It should never be forgotten in interpreting ancient
sites that such things were equally “practical” to early technologists as ore
and fuel quality or furnace design.
Furnace repair:
Before each smelt the tap arch
was rebuilt with loam “bricks” and sealed to prevent gas leaks from
occurring. No other repairs were made to the lining of the furnace.
Product:
Of the four smelts, two
produced successful blooms. Both used bog ore enriched with magnetite. These
blooms were roughly lens shaped (plano-convex),
with the more convex surface oriented downwards in the furnace. It was this
convex surface which was less densely metallic, including more slag, imbedded
charcoal, and casts of charcoal pieces that had burned to ash. The flatter
and uppermost surface was densely metallic by comparison. The first bloom
weighed seven kilos and was cut into two major pieces. The second bloom was
sectioned through after I returned to Buffalo,
New York using an industrial power
hacksaw. Interestingly, large interior voids in this bloom were mostly empty
rather than being filled with slag as expected. The bloom was easy to cut and
did not destroy the hacksaw blade. Spark testing on a grinding wheel showed
the starburst, secondary sparking characteristic of high carbon material,
specifically steel, on some exterior zones, but the interior produced the
long, non-branching sparks typical of wrought iron. The slag was a dense,
black fayalite-type slag with a metallic sheen.
Interior voids showed tabular crystals, most likely Wustite
growing on the interior surfaces, surrounded by glassy vitreous material.
Analysis:
Metallographic
Two areas of the bloom were
identified as wrought iron and steel respectively based on spark testing
using a grinding wheel. Small sections were cut from these areas of the
bloom, mounted in epoxy resin, and ground and polished to 1μm with
diamond paste. The samples were etched in 2% Nital
for 30 sec to enhance the microstructure of the metal. Reflected light
microscopy was used to look at the microstructure and the entrapped slag for
identifiable features and to better understand the blooms production.
All of the structures seen
were typical of slow cooling, most likely a result of the heat-holding
ability of the large iron mass. The “wrought iron” section (Sample 1), taken
from the top part of the bloom showed a structure consisting almost entirely
of ferrite, with slag inclusions of variable size. In some areas close to the
surface, a fine dendritic pearlite
formation surrounded by a ferrite matrix was observed. The “steel” section
(Sample 2), taken from the underside of the bloom showed a mixed structure of
pearlite and ferrite grains surrounded by
relatively larger slag inclusions. In some areas of this section, the pearlite content was approximately 50%, indicating
estimated carbon content for the entire area of about 0.4% carbon. Also of
interest was the widmanstätten structure of the pearlite in some areas. This structure has been observed
in experimental blooms by other researchers (Salter and Crew 1997), and is
also generally associated with slow cooling. The steel region, while a hypoeutectoid steel,
would still be possible to harden by non-equilibrium heat treating
(“quenching”). The slags were fayalitic
throughout, with a dendritic growth of wustite formed in a fayalite
matrix on some of the larger inclusions.
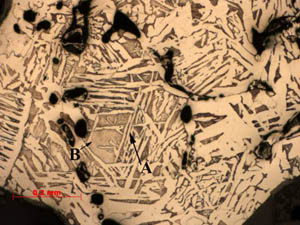
 Figure
1: Sample 2: This sample is more
complex than sample 1 with several areas of varying compositions. This area is a hypo-eutectoid steel with a
majority of large ferrite grains (A) and smaller pockets of pearlite (B). Figure
1: Sample 2: This sample is more
complex than sample 1 with several areas of varying compositions. This area is a hypo-eutectoid steel with a
majority of large ferrite grains (A) and smaller pockets of pearlite (B).
click on images to view at full size
 Figure
2: Sample 2: This area is
comprised of both primary and secondary widmanstatten
ferrite growth (A) and pearlite (B). This unique structure forms from austenite
at relatively high temperatures and consists of ferrite and pearlite. The
cross-hatched appearance is due to the ferrite having formed along specific
crystallographic planes. Figure
2: Sample 2: This area is
comprised of both primary and secondary widmanstatten
ferrite growth (A) and pearlite (B). This unique structure forms from austenite
at relatively high temperatures and consists of ferrite and pearlite. The
cross-hatched appearance is due to the ferrite having formed along specific
crystallographic planes.
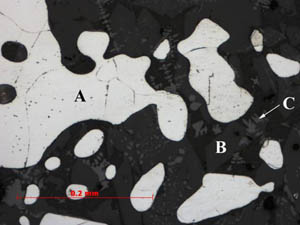 Figure
3: Sample 2: A third area within sample 2 is a dense fayalitic
slag region. The underbody of fayalite (B) has overgrowth of wustite
(C) and ferrite iron (A). It is a low carbon region of this sample. Figure
3: Sample 2: A third area within sample 2 is a dense fayalitic
slag region. The underbody of fayalite (B) has overgrowth of wustite
(C) and ferrite iron (A). It is a low carbon region of this sample.
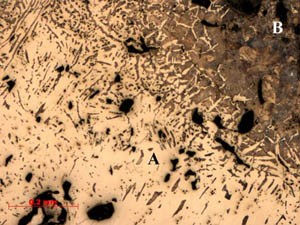 Figure
4: Sample 1: This sample is composed of porous ferritic
iron (A) with smaller pockets of dense fine pearlite
(B). Figure
4: Sample 1: This sample is composed of porous ferritic
iron (A) with smaller pockets of dense fine pearlite
(B).
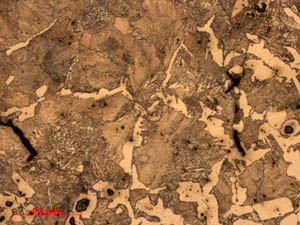 Figure
5: Sample 1: Close up of the finely dispersed pearlite
with some intergranular ferrite. The sample is a hypo-eutectoid steel. Figure
5: Sample 1: Close up of the finely dispersed pearlite
with some intergranular ferrite. The sample is a hypo-eutectoid steel.
X-ray fluorescence
The polished sections were
analyzed using X-ray fluorescence. A Bruker ARTAX
u-XRF was used for the analysis. The system is equipped with a helium purge
and variable collimators from 0.2 mm to 1.5 mm. For this study a 0.65 mm
collimator was used and the machine was run with the helium purge to help
identify any lighter elements that might be preset in the metal.
Samples were run for 90 sec at 50 KeV and 700 uA. The ARTAX does not presently have a fundamental
parameters programming which would give relative values for those elements
present in the metal. This machine must be calibrated for specific compositional
analysis to give any real indication of compositional values. It is
unpractical to do this for each ‘unique’ material that comes into our lab,
but the resulting spectra accumulated from the analysis can be interpreted
qualitatively, and as such, the particular elements present in the metal can
be identified and roughly estimated for their concentrations as major, minor
or trace levels.
Analysis showed the iron to be
relatively pure with limited trace elements including Copper, Arsenic and
nickel. Interesting, the nickel only appears in the pearlite
phase of the metal. No obvious explanation presents itself.
The individual smelts:

Four separate smelts were
carried out using this furnace. The first smelt proceeded essentially as
described, and produced a good bloom. The second was carried out at the
request of, and with the assistance of Arne Espelund,
to test his hypothesis that iron could be smelted from bog ore and iron-rich
slag. This smelt failed to produce good malleable iron, and will be left for
Dr. Espelund to describe. The third smelt was the
more successful of the two carried out according to the description already
given. The last and final smelt was carried out with Thijs
van de Manakker, in order to test whether or not
good iron could be produced from the German ore using a furnace that had
already proven successful. It failed to produce a bloom of malleable iron.
The first smelt:
This initial experiment was
carried out on Friday September 15th, commencing with ore charging
at 15:15 and ending at 22:11, for a final duration of 7 hours.
Charcoal was consumed at the rate of 4kg/hr, with 28 kg consumed during the
actual smelt. The furnace was charged with ore at the same time that charcoal
was added. The ore used was a 50/50 (wt.) mixture of the German bog ore and
hammer scale. The final bloom weighed 8 kg.
Third smelt:
The third smelt was carried
out on Sunday September 17th, with the first ore added at 10:30, and the bloom extracted soon after 16:20, for a duration
of almost six hours. The same ore mixture was charged at a rate of 6kg/hr.
Total charcoal consumption was 36kg. Ore
consumption was 34kg. The final bloom weighed 10kg after consolidation.
Discussion:
It seems clear that the
decision to enrich the bog ore with magnetite hammer scale largely accounted
for the success of the two smelts which produced good iron in quantity. The
third smelt though seems to have benefited from other factors. It was faster,
consuming charcoal more quickly, and since the ore was always charged in
equal parts by weight with the charcoal, the whole smelt was of shorter
duration. It seems likely that the fire burned faster and hotter due to the
furnace already being hot and dry at the outset. Efficiency was probably also
improved by the patching of cracks after the initial smelt. If the point of a
shaft furnace is to contain reducing gasses for a longer period of reaction
time, then large cracks shooting out blue flame would seem to be a likely
explanation for reduced efficiency. There was no apparent side-wall
leakage during the third smelt. The yield was a remarkable bloom of ten
kilograms, a size and weight from 34 kilograms of ore that shows particularly
efficient extraction for a furnace of this type- at least in modern times.
References:
Craddock, Paul. 1995. Early
Metal Mining and Production. Washington DC:
Smithsonian Institution Press.
Crew, P. and Charlton, M.
2005. “The anatomy of a furnace.” Conference paper presented at Metallurgy-a
Touchstone for Cross-cultural Interaction. London:
British Museum.
Salter, C. and Crew, P. 1997.
“high phosphorus steel from experimentally smelted
bog-iron ore.” In Early Ironworking in Europe
and international conference at Snowdonia National
Park Study Center, Wales.
|